* Dr. Androulla N. Miliotou
In recent days an excellent news has appeared in the press, in the TV news and on various sites and you may have noticed the reference to CAR immunotherapy. But what is this technology about? It has nothing to do with a simple car, but it is the treatment that has the greatest potential to lead humanity to a world without cancer!
On February 2, 2022, the publication of the scientific paper in the internationally renowned journal Nature was made by the team of the American Professor of Immunotherapy Carl H. June, from the University of Pennsylvania. June is considered the "father" of the treatment that utilizes CAR T-lymphocytes against cancer.
What exactly is so promising regarding CAR immunotherapy against cancer?
Cancer is one of the leading causes of death worldwide, resulting in the constant search for new therapeutic approaches. In recent years, scientists' interest has been geared towards immunotherapy. The immune system initially recognizes cancer cells, but they eventually escape immune surveillance, due to the "evolutionary pressure" exerted and leading to the development of escape mechanisms (1). Immunotherapy seeks to make cancer cells visible, making it easier for our immune system to destroy them (2).
Chimeric Antigen Receptor (CAR) therapy (3) is a personalized therapy that involves the genetic modification of individual patient's immune system cells to selectively target cancer cells (4). In other words, we employ our own immune system to "fight" cancer cells only, recognizing them as "enemies", while not affecting healthy cells at all.
The genetic modification of immune cells, usually T-lymphocytes, is made to express the CAR receptor, which has the ability to recognize a cancer antigen on the surface of cancer cells (regardless of the histocompatibility system). In fact, the CAR receptor can identify 'labels' (i.e., the antigens) that only carry cancer cells, so it can identify them.
Subsequently, genetically modified T-lymphocytes are cultured ex vivo, i.e. removed from the patient and processed in a laboratory, for further development (expansion). Prior to subsequent infusion of CAR T-lymphocytes back to patients, lymphocatastrophic chemotherapy is required to enable the establishment and persistence of the infused CAR T-lymphocytes in the circulation for a longer period of time and/or to reduce the anti-CAR immune response (5).
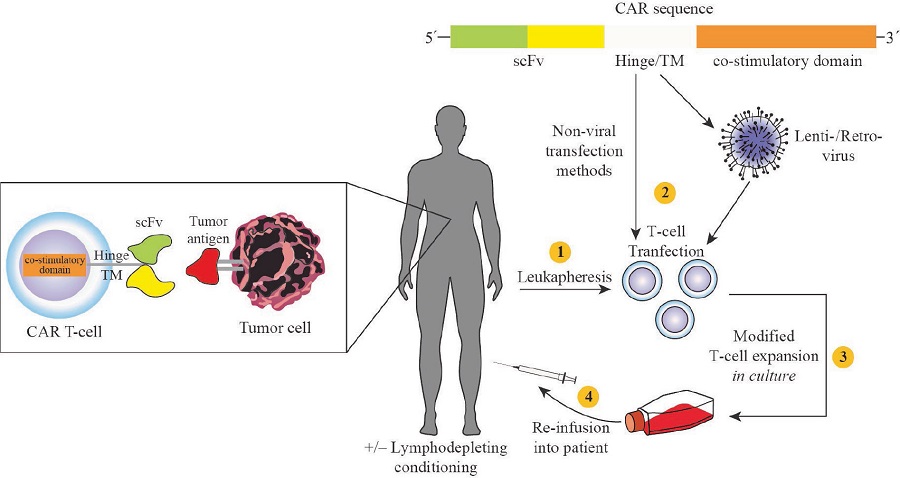
Figure 1: Schematic representation of CAR immunotherapy (Miliotou, A. N., et al., Current pharmaceutical biotechnology 2018, 19 (1), 5-18).
If we would like to simplify the idea around CAR immunotherapy, we could say that:
1. The patient's immune cells (T-lymphocytes) are selected and removed by leukapheresis. In our immune system, T-lymphocytes, either alone or in collaboration with other cells, destroy germs or cancer.
2. T-lymphocytes are genetically engineered to express the CAR receptor on their surface and to enhance their ability to more accurately identify cancer cells.
3. CAR T-lymphocytes are injected back to the patient.
4. The CAR receptor can distinguish cancer cells, since it is designed to bind to cancer antigens, i.e. small protein labels.
5. After the CAR is attached to the antigen ("key in the lock") the immune detects the cancer cell and is recruited to destroy it.
The genetic processing of immune cells, in order to express the CAR receptor, is done mainly by using the technology of delivering genetic material, via lentiviruses, while there are several advanced studies that utilize the mRNA technology now known to all of us (6). Our research team, under the supervision of Dr. Papadopoulou Lefkothea, Professor of Pharmacology AUTh, utilizes mRNA technology for the development of CAR immunotherapy against oral cancer, with very encouraging results. The delivery of mRNA is conducted through the innovative platform using Protein Transduction Technology, which has been patented nationally (DE 1010063; international patent-pending PCT/GR2020/000059).
Clinical trials to date have demonstrated spectacular efficacy in patients with End Stage Acute Lymphoblastic Leukemia (all) (7) using anti-CD19 CAR T-lymphocytes, with 92% full recovery. Tisagenlecleucel-T (Kymriah/Novartis) corresponds to the first approved CAR T-treatment and is intended for children and young adults suffering from recurrent/refractory ALL. The product has been approved by the FDA (30-8-2017) (cost: $475,000) and approved by the EMA (October 2018). Axicabtagene-Ciloleucel (Yescarta/Kite Phama) was approved by the FDA (18-10-2017) (cost: $373,000) and is intended for patients with relapsed/refractory non-Hodgkin's B-lymphocyte lymphoma (NHL) who are not eligible for autologous stem cell transplantation (5, 8).
CAR treatments have already been performed in patients, children and young adults in Greece, with relapsed or refractory Acute Lymphoblastic Leukaemia and in patients with Diffuse B-cell Lymphoma, in the Children's Hospital and the Evangelismos Hospital in Athens, and in the Papanikolaou Hospital in Thessaloniki. The first patient treated with CAR T-lymphocytes was Emily Whitehead, when she was 6 years old (back in 2011) and faced a life-threatening relapse of acute lymphoblastic leukaemia, the most common childhood cancer. Emily's cancer resisted 16 months of chemotherapy when they told her parents that her cancer had come back and that she had no chance of surviving. Determined to save their child's life, her parents enrolled their daughter in a clinical trial of a new therapy at the time, the CAR immunotherapy that was designed to turn Emily's immune system into a powerful cancer weapon. The treatment worked and the cancer fell into complete remission (9). Emily in the year 2022 is going through the end of her adolescence and full of health and appetite for life, while a few years ago it was a matter of whether she wouldsurvive for a few months.
The man who led the clinical team and who designed the protocol that saved Emily's life was none other than Carl June. And maybe Patient 1 was Emily, but many others followed. Today, about 140 pharmaceutical/biotechnology companies are developing immunotherapy CAR programs.
The recent work of the June team in Nature has come to give hope and a revival of morale to the global scientific community. He mentions two of the first patients (Doug Olson and Bill Ludwig) with chronic lymphocytic leukaemia, who were infused with CAR T-lymphocytes in 2010 as part of a phase I clinical trial and responded with complete remissions of the cancer (“cancer-free”), while even more impressive is that persistence of infused, functional CAR T-lymphocytes was also observed so many years after treatment. This finding yields the long-term potential of CAR T-lymphocytes, which appear to have saved and will be able to save a large number of patients with terminal and non-terminal blood cancer. In Olson, the remaining CAR T-cells are all descendants (clones) of three single CAR T-lymphocytes. If scientists are able to understand what made these cells function, fewer, more efficient cells could be injected into patients in the future, and this will reduce the cost of treatment. In addition, a burning issue is the utilization of CAR immunotherapy in solid tumors, with clinical studies being conducted with excellent remission results.
Is CAR Immunotherapy the Future to Eliminate Cancer? It appears to be a powerful weapon in our quiver. The only sure thing is that science is evolving rapidly and the publication of Carl June and his team from the University of Pennsylvania is a milestone in the fight against cancer science. Closing only a smile is formed by Doug Olson's words:
“That day in 2010, when I infused myself with my CAR T-lymphocytes and my cancer cells disappeared – it meant that there was a whole new treatment. This gives cancer patients hope. And if there is no cure for them today, there is a chance that the cure is very close. "
1. Dunn, G. P., et al., Annu Rev Immunol 2004, 22, 329-60.
- Haji-Fatahaliha, M., et al., Artif Cells Nanomed Biotechnol 2016, 44 (6), 1339-49.
- Gross, G., et al., Proc Natl Acad Sci U S A 1989, 86 (24), 10024-8.
- Bridgeman, J. S., et al., Curr Gene Ther 2010, 10 (2), 77-90.
- Miliotou, A. N., et al., Current pharmaceutical biotechnology 2018, 19 (1), 5-18.
- Miliotou, A. N. and Papadopoulou, L.C. Chimeric Antigen Receptor T Cells 2020, 87-117
- Kochenderfer, J. N., et al., Journal of clinical oncology: official journal of the American Society of Clinical Oncology 2015, 33 (6), 540-9.
- Milone, M. C., et al., Molecular therapy: the journal of the American Society of Gene Therapy 2009, 17 (8), 1453-64.
- https://www.cancerresearch.org/en-us/immunotherapy/stories/patients/emily-whitehead
* Dr. Miliotou is the Program Coordinator of the Accredited – Certified Programs of Study "Medical Representatives Management (4 years/Bachelor)" and "Pharmacy Assistants (Technicians) (2 years/Diploma)" of KES College. In her PhD thesis, since 2016, she has been involved in the successful development of an innovative intracellular delivery platform for mRNA. In 2020, the results of her research activity, regarding mRNA technology, have been approved for national patenting and have been filed at international level.




 Share On Facebook
Share On Facebook Share On Twitter
Share On Twitter Share On Linkedin
Share On Linkedin
